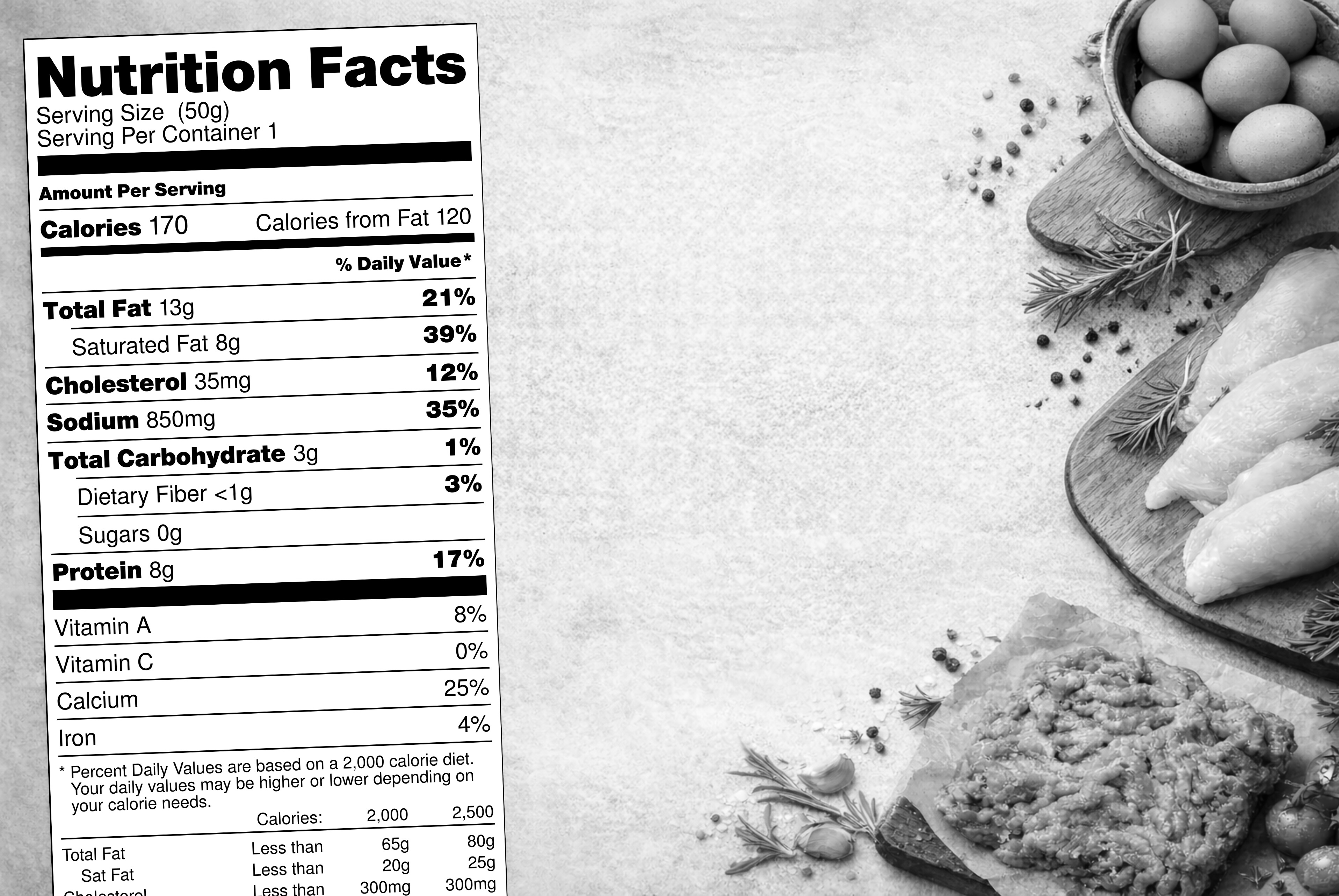
5 Reasons R&D and Regulatory Teams Need Shared Data
Spreadsheets are everywhere in food and supplement R&D, and for good reason. They’re flexible, familiar, and incredibly powerful for quick calculations, modeling, and experimentation.
But as teams scale, portfolios grow, and regulatory expectations tighten, spreadsheets alone can’t carry the full weight of product development and labeling.
The challenge isn’t that spreadsheets are “bad.” It’s that they were never designed to be a shared system of record for formulation, ingredients, and labels across R&D and Regulatory.
When critical data lives in separate files, manual workflows, and email threads, even strong teams struggle to move fast while staying aligned and compliant.
This is why more modern product organizations are shifting from spreadsheet-first to shared-data-first workflows.
1) Spreadsheets are great for analysis, not as your single source of truth
Spreadsheets shine when you’re exploring ideas, running scenarios, or stress-testing assumptions.
Where they struggle is when they become the official record for:
- Final formulas
- Approved ingredients
- Supplier specs
- Label calculations and changes
- Tracking ingredient changes
- Regulatory documentation
A single broken formula, overwritten cell, or mistaken value can quietly change nutritionals, and those changes are hard to trace once data moves between multiple files.
The risk increases when teams must manually copy data from spreadsheets into other systems. Each handoff adds friction and the potential for human error - not because people are careless, but because manual processes are inherently fragile.
2) Manual ingredient data entry steals time from real innovation
Most formulators didn’t choose R&D to spend hours retyping or reconciling data.
Yet many teams still spend significant time:
- Cleaning up formula versions
- Manually updating ingredient nutritionals
- Fixing broken spreadsheets
- Re-entering data into labeling tools
- Chasing answers to “Is this the latest version?”
None of this creates better products - it just keeps projects moving.
When ingredient data can flow automatically from specs into formulas and labels, teams reclaim time for what actually matters: creativity, optimization, and problem-solving.
3) Spreadsheets work early, but get disconnected at scale
Spreadsheets can be perfectly adequate when you have:
- A small portfolio
- A handful of suppliers
- A tiny team
But as you grow, data often splinters across:
- Nested folders
- Duplicated files
- Confusing version names
- Endless tabs
- Shared drives no one fully owns
Finding the “latest” formula or approved ingredient list becomes a scavenger hunt - not a workflow.
The issue isn’t the spreadsheet itself; it’s the lack of structure around how data is stored, connected, and governed.
4) R&D, Regulatory and QA teams need to see the same data
In many organizations:
- Procurement and QA manage their own ingredient list
- R&D tracks formulations and ingredients in a separate set of files
- Regulatory references yet another set of files
- Labeling lives somewhere else entirely
This fragmentation creates misalignment, rework, and last-minute scrambles.
Modern teams benefit from a shared data layer where:
- Ingredient specs feed directly into formulas
- Formulas feed directly into labels
- Regulatory can review in context - not just via screenshots or PDFs
When everyone works from the same data, handoffs become smoother, decisions become clearer, and the risk of error decreases significantly.
5) Purpose-built platforms bring the food industry into the future
Leading teams are not abandoning spreadsheets - they’re using them more intentionally.
Spreadsheets remain great for:
- Prototyping
- Scenario modeling
- Quick calculations
But the system of record shifts to a purpose-built platform that provides:
- Structured ingredient data
- Version-controlled formulas
- Automated nutrition calculations
- Traceability from ingredient to label
- Clear visibility for R&D and Regulatory
In this model, spreadsheets complement your core system - they don’t replace it.
When is it time to evolve beyond spreadsheets alone?
You may be ready for a shared data system when:
- You spend too much time reconciling versions and copy-pasting
- Data moves manually between teams or tools
- Traceability is difficult to reconstruct
- Handoffs between R&D and Regulatory feel brittle
- Your portfolio is growing faster than your processes
At that point, the question isn’t whether spreadsheets are useful - it’s whether they’re sufficient.
Spreadsheets + shared data = the modern workflow
Spreadsheets aren’t going anywhere, nor should they.
The difference today is that high-performing teams pair them with a connected data backbone that supports collaboration, compliance, and scale.
Platforms like ENTR bring structure, automation, and traceability to formulation and nutrition labeling, while still giving teams the flexibility they value.
The result? Faster launches, fewer mistakes, and clearer alignment between R&D and Regulatory.